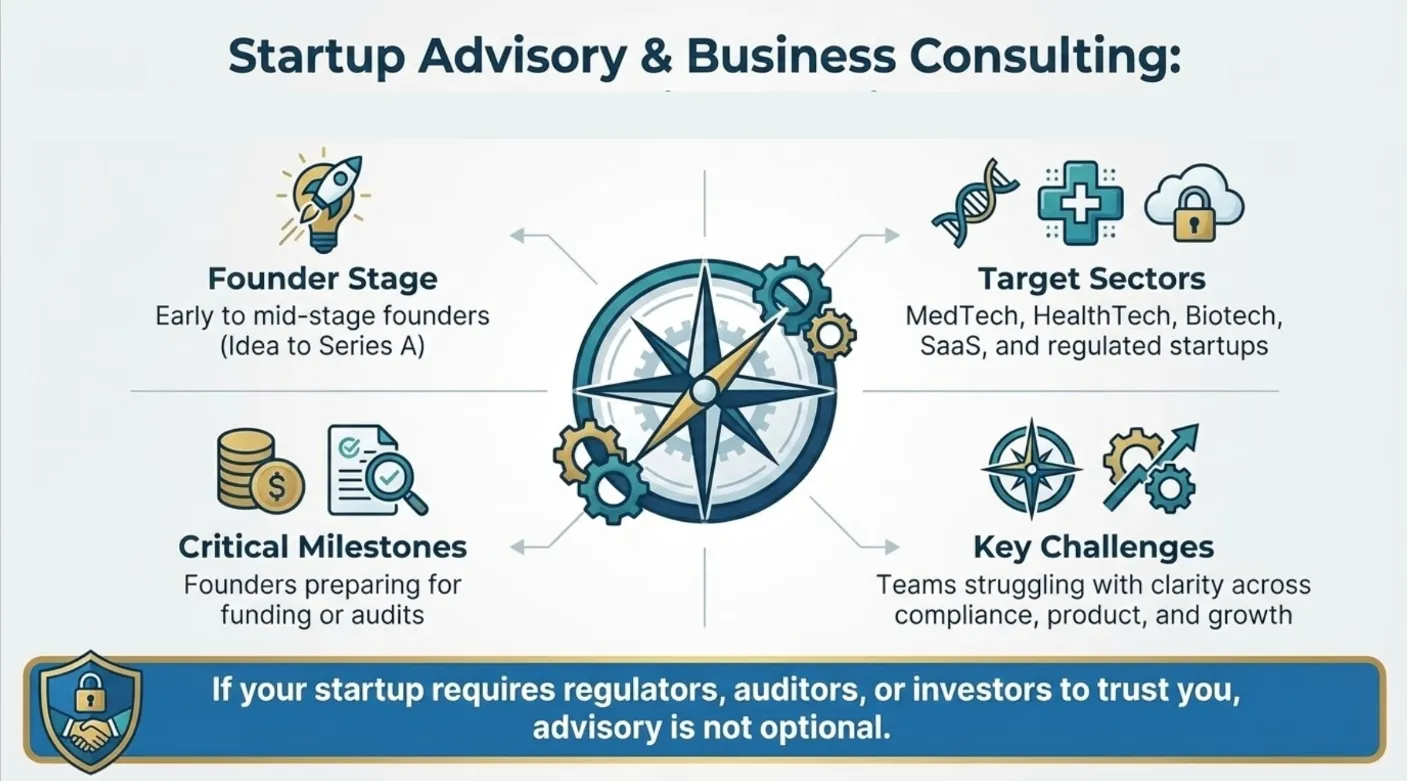
Startup Advisory & Business Consulting in India
Between incorporation, compliance, funding pressure, regulatory uncertainty, and investor expectations, most founders are forced to make decisions before they fully understand the consequences. This is where a startup business
Read More