Key Cybersecurity Documents for SaMD FDA 510(k) Submissions
As Software as aMedical Device (SaMD) continues to evolve, cybersecurity is no longer optional— it’s a regulatory imperative.
For FDA 510(k) submissions, manufacturers must demonstrate a robust security framework throughout the software lifecycle
Core Cybersecurity Documents Required for SaMD FDA 510(k) Submissions






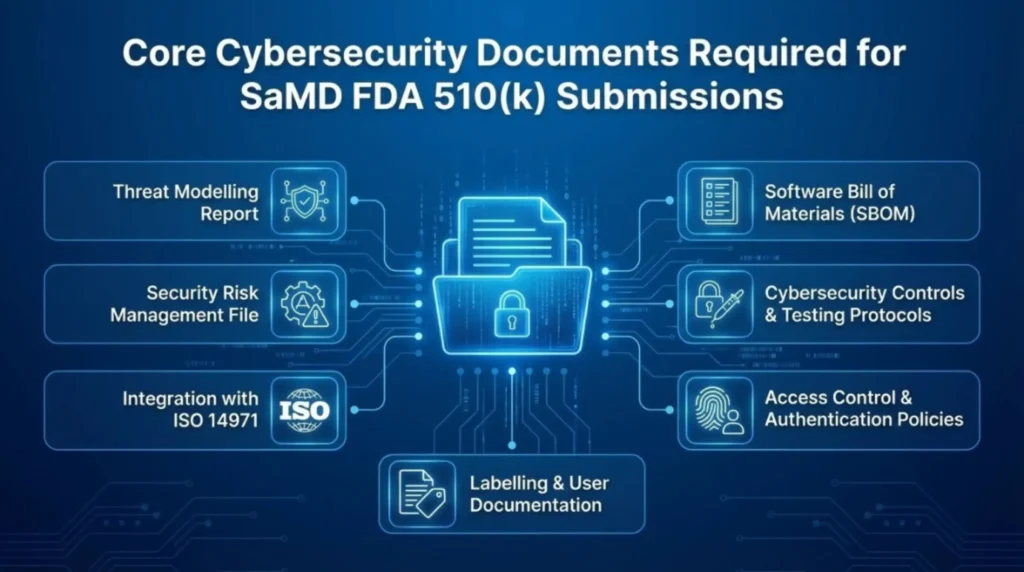
Why These Cybersecurity Documents Are Critical for FDA 510(k) Approval
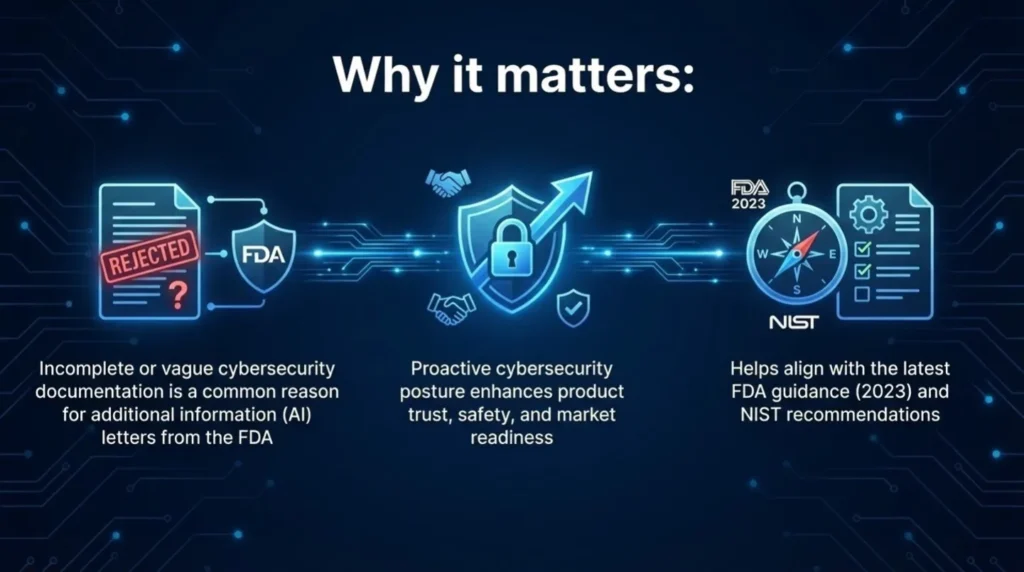
Incomplete or vague cybersecurity documentation is one of the fastest ways to trigger FDA Additional Information letters. That means delays, rework, and a very awkward call with your regulatory team.
A strong, well-documented cybersecurity posture shows the FDA that your SaMD is designed for real-world risk. Not just clinical use, but threats, failures, and updates over time. It builds trust, protects patient safety, and keeps your 510(k) review on track.
More importantly, it aligns your submission with the latest FDA cybersecurity guidance from 2023 and NIST expectations.
Translation. Fewer surprises, faster reviews, and a product that is actually ready for the market, not just approved on paper.
At D2R Global Consulting, we help SaMD innovators build 510(k)-ready cybersecurity documentation that meets FDA expectations—from threat modeling to SBOMs and postmarket controls.
Planning a 510(k) for your SaMD?
Let’s make cybersecurity your submission strength.



Comments are closed