Introduction
Whether you’re pursuing regulatory approval in the United States (US FDA) or the European Union (CE marking), biocompatibility testing is a non-negotiable requirement for medical devices that come into direct or indirect contact with the human body.
At D2R Global Consulting, we help device manufacturers navigate the complexities of international biocompatibility standards to ensure both compliance and patient safety.
Key Regulatory Frameworks
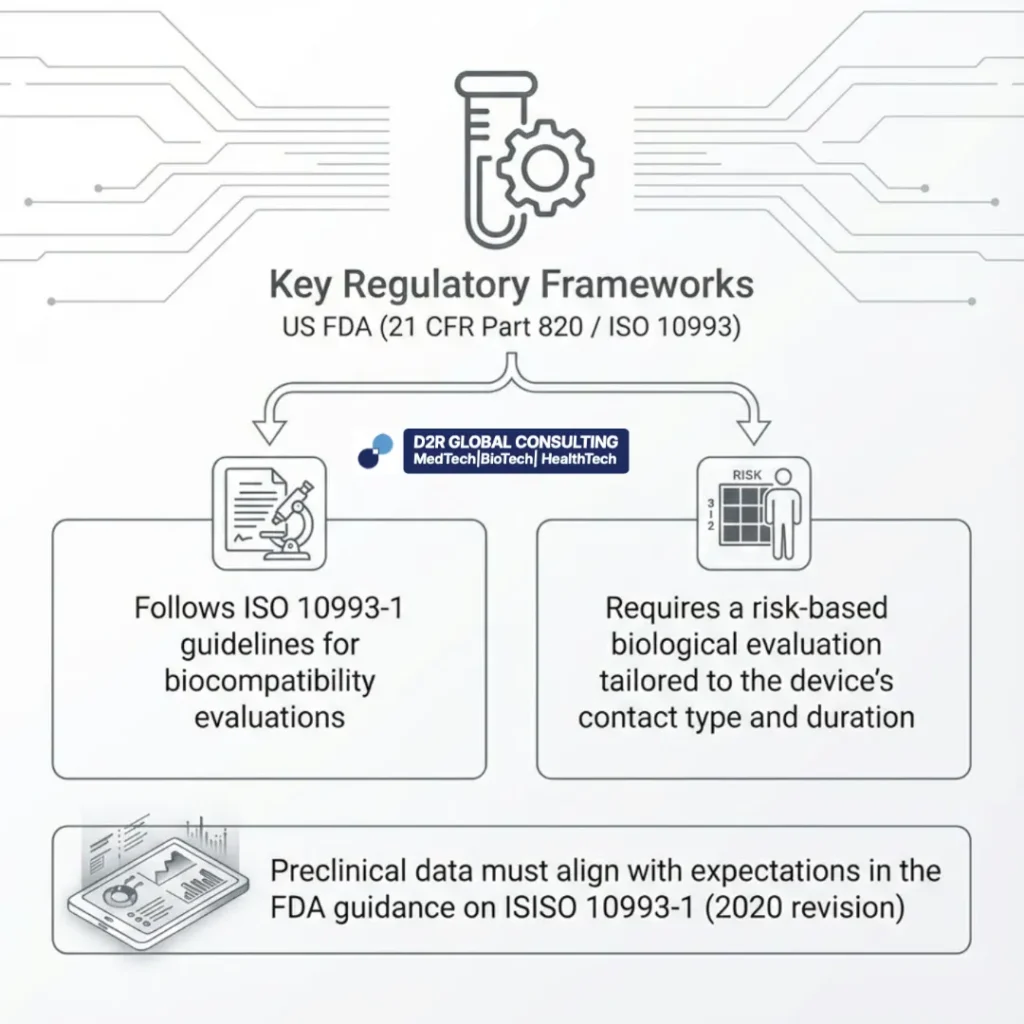
US FDA (21 CFR Part 820 / ISO 10993)
Follows ISO 10993-1 guidelines for biocompatibility evaluations
Requires a risk-based biological evaluation tailored to the device’s contact type and duration
Preclinical data must align with expectations in the FDA guidance on ISO 10993-1 (2020 revision)
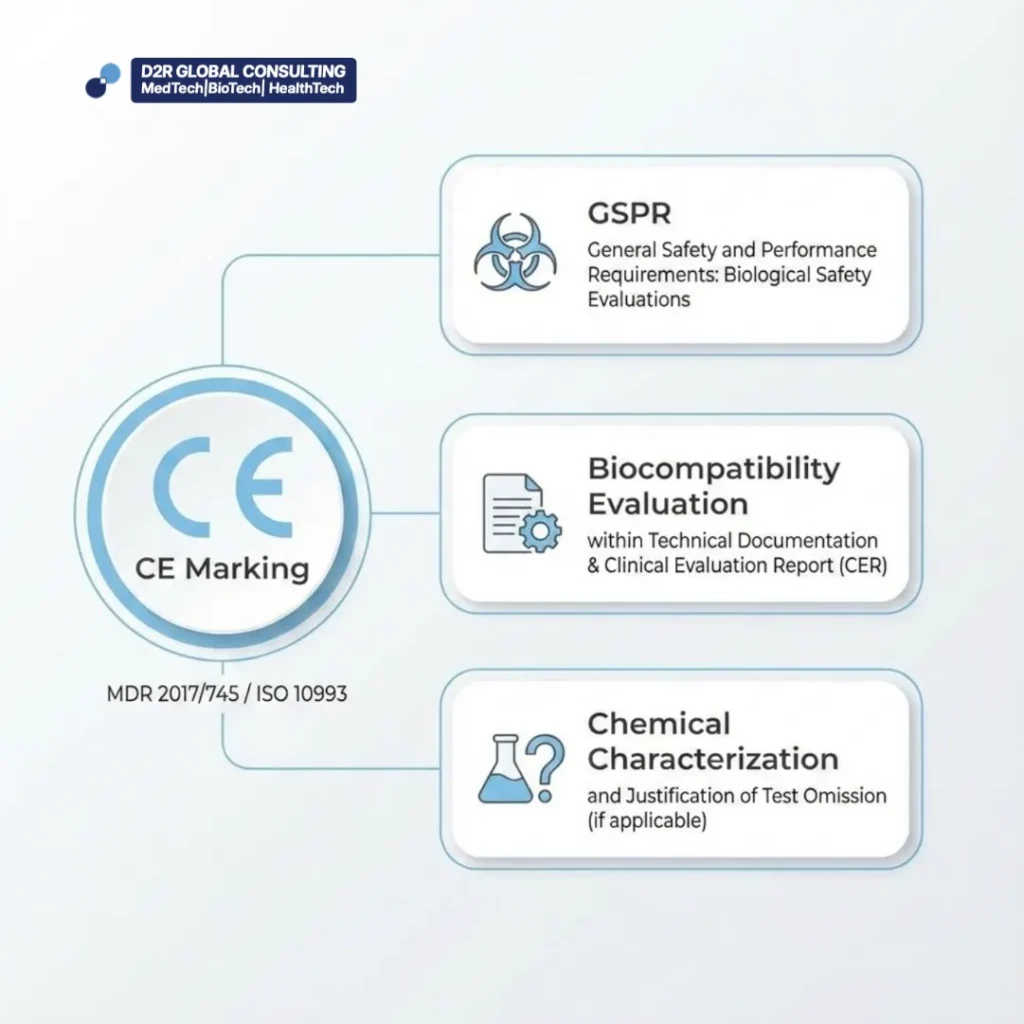
CE Marking (MDR 2017/745 / ISO 10993)
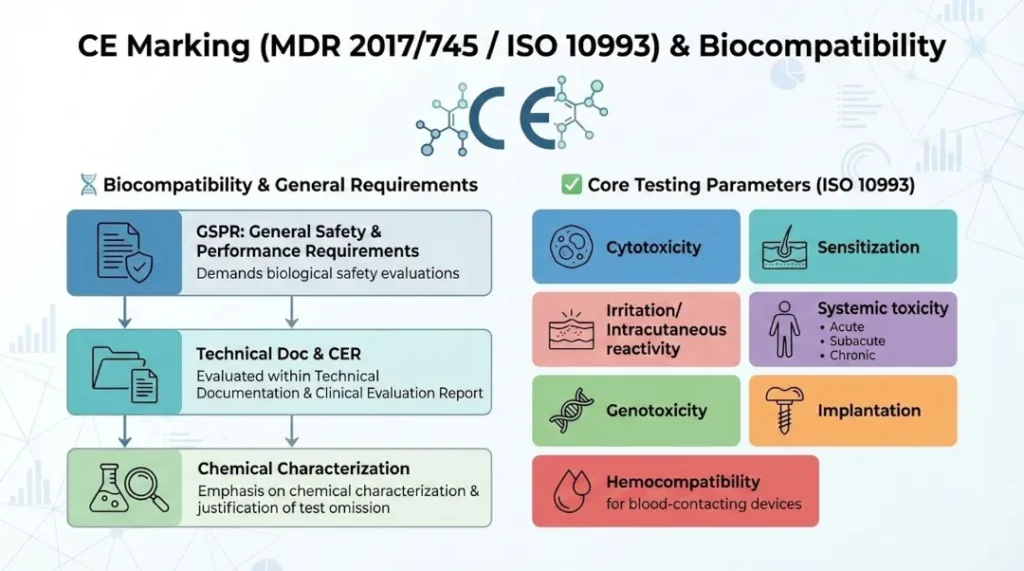
Demands biological safety evaluations as part of the General Safety and Performance Requirements (GSPR)
Biocompatibility is evaluated within the technical documentation and clinical evaluation report (CER)
Emphasis on chemical characterization and justification of test omission, if applicable
Core Testing Parameters (based on ISO 10993 series):
Cytotoxicity
Sensitisation
Irritation/Intra-cutaneous reactivity
Systemic toxicity (acute, subacute, chronic)
Genotoxicity
Implantation
Hemocompatibility (for blood-contacting devices)
Cytotoxicity
Cytotoxicity testing evaluates whether device materials cause cell damage or death when in contact with living tissue. It is often the first biological test performed under ISO 10993-5. For example, a catheter material is exposed to cultured cells to assess viability. A failed cytotoxicity test can halt development early, saving time and cost before advanced testing begins.
Source: https://www.iso.org/standard/36406.html
Sensitisation
Sensitisation testing determines whether repeated exposure to a device material can trigger allergic reactions. This is especially critical for devices with prolonged skin contact, such as wearable sensors. For example, adhesives used in ECG patches are evaluated to ensure they do not cause delayed hypersensitivity. Regulators expect clear justification when sensitisation testing is omitted.
Source: https://www.iso.org/standard/44908.html
Hemocompatibility (for Blood-Contacting Devices)
Hemocompatibility testing assesses how a device interacts with blood, focusing on risks like clotting, hemolysis, or platelet activation. This is critical for devices such as stents or dialysis tubing. For example, blood-contacting polymers are tested to ensure they do not trigger thrombosis. Weak hemocompatibility data almost always attracts FDA scrutiny.
Source: https://www.iso.org/standard/68450.html
Genotoxicity
Genotoxicity testing determines whether device materials can damage genetic material, potentially leading to mutations or cancer. This is especially relevant for long-term implants or polymer-based devices. For example, residual manufacturing chemicals are assessed for DNA-damaging potential. FDA reviewers closely examine genotoxicity rationales, especially when testing is waived.
Source: https://www.iso.org/standard/63610.html
Systemic toxicity testing evaluates whether substances released from a device can cause harmful effects throughout the body. The duration depends on device contact time. For example, an implantable pump may require chronic toxicity data due to long-term exposure. Skipping or under-justifying systemic toxicity is a common reason for regulatory pushback.
Source: https://www.iso.org/standard/68936.html
Irritation / Intra-cutaneous Reactivity
Irritation testing assesses localized inflammatory responses following direct contact with device materials. It helps identify short-term tissue reactions such as redness or swelling. For instance, injection ports or surface coatings are tested using intracutaneous injections in controlled models. Even low-risk devices can trigger FDA questions if irritation risks are not addressed clearly.
Source: https://www.iso.org/standard/36407.html
Implantation
Implantation studies evaluate the local tissue response to a device when placed inside the body. These tests assess inflammation, fibrosis, and integration over time. For example, orthopedic screws are implanted to observe tissue compatibility at the implantation site. Results help confirm that the device performs safely in its intended anatomical location.
Source: https://www.iso.org/standard/36409.html



Comments are closed